A Beginner’s Guide to Polyamines
What are they?
Polyamines are short organic molecules which have at least 2 amino (-NH2) groups. They are found in both bacteria and eukaryotes, and are ubiquitously expressed in cell types, and are likely to be important for basic cellular functions. Polyamines are able to bind to a variety of cellular components, such as DNA, RNA and proteins. The function of polyamines is not fully understood, although they are known to play a variety of roles, including in DNA repair and ion channel modulation. They are known to influence the permeability of the blood-brain barrier and they also have a major role in the area of translation, with these molecules being key regulators of mRNA translation elongation, as well as possible roles in both translation initiation and termination. The most important polyamines in eukaryotes are putrescine, spermine, spermidine.
How are they made?
In eukaryotes, polyamines are synthesised from the precursor non-proteinogenic amino acid ornithine. It is converted to the first well-known polyamine, putrescine, by the actions of ornithine decarboxylase (ODC) (1). From here, for the formation of spermidine and spermine, there is a need for another molecule, namely S-adenosylmethioninamine. It is formed through the decarboxylation of S-adenosyl methionine by the enzyme adenosylmethionine decarboxylase, or AdoMetDC (AMD1), leaving a propylamine group attached. It is this propylamine group that is key for the formation of both spermidine and spermine. Both spermidine synthase and spermine synthase employ a mechanism to transfer propylamine from S-adenosylmethioninamine to their pertinent substrate of interest (these being putrescine for spermidine synthase, and spermidine for spermine synthase). Thus, the final two polyamines in this process (spermidine and spermine) are synthesised.
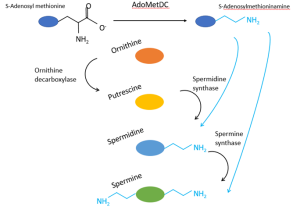
Figure 1: Canonical pathway of polyamine biosynthesis in eukaryotes
Translation
Polyamines play major roles in multiple facets of translation, from general stimulation to maintenance of translational fidelity. Polyamines bind to ribosomes in large numbers (of up to 500 per ribosome) (2), although only a small number bind to sites known to be important for ribosomal function (3). Multiple studies have linked polyamine to stimulation of translation, with authors finding that polyamine supplementation to cell-free extracts lowered the amount of Mg2+ ions needed for optimum translational activity (4, 5). Furthermore, polyamine supplementation exhibited no bias for particular transcripts, indicating its activity was based on direct interactions with the translational machinery (6).
What are they?
- eIF5A
The best understood role of polyamines is with regard to eIF5A. eIF5A is a translation factor which has role for the resolution of hard-to-translate polyproline motifs (7), and has also been implicated in translation termination (8). Spermidine acts as an initial donor molecule in the first step of this hypusination of eIF5A. This is a unique post-translational modification of a conserved lysine residue in the eIF5A protein by a series of enzymatic reactions (9).
In vitro assays also reveal a curious functional overlap between eIF5A and polyamines. The addition of eIF5A (regardless of hypusination) was sufficient to restore translational activity caused by the absence of polyamines. eIF5A binds in the ribosomal E-site (10) and is thought to interact with both the body and acceptor stem of the P-site tRNA. It, therefore, seems reasonable to expect that polyamines may also interact with the peptidyl-tRNA, at least in eukaryotes.
- Elongation
However, a question remains. By what mechanism do these molecules stimulate translation? While translation initiation is generally seen as the bottleneck in protein synthesis and thus the major process governing translational production, polyamines appear to have a greater role in stimulating translation elongation. Interestingly, early research utilising the Met-puro assay came to the conclusion that polyamine treatment increased the formation of initiation complexes and thus acted through stimulation of this process (11). This contrasted with other work of the era, which demonstrated that polyamines upregulated the incorporation of amino acids into extending peptides, as opposed to those that are newly initiated (12). However, later investigation into the details of the Met-puro assay determined that it reports on ribosomal peptidyl-transferase activity (a measure of elongation), whereas previously it was thought to function as an indicator of first peptide bond formation (i.e., initiation). More recent evidence has bolstered the role of polyamines in elongation, with polyamine treatment being found to accelerate the process of codon recognition without impacting on decoding fidelity (13).
- Initiation
Yet, it’s not all about elongation. More recent studies have also demonstrated an additional role for polyamines in initiation. A 2010 study investigating mitochondrial protein synthesis found that spermine treatment resulted in an increased inhibition of dissociation of mitochondrial initiation factor 3 from the 55S ribosome, while also promoting fMet- tRNA binding to ribosome subunits (14).
Additionally, depletion of polyamines led to a decrease in the amounts of large polysomes, indicative of a reduction in initiating ribosomes (15). Evidence for a mechanism underlying these effects was uncovered when it was discovered that polyamine depletion activated the PERK, a key enzyme in activation of the stress response. This activation in turn phosphorylates and inhibits the translation initiation factor eIF2α, supressing initiation (16). Thus, it is clear that polyamines influence multiple parameters of translation.
Cancer
It has been shown that increased intracellular polyamine concentrations are associated with cell proliferation and tumorigenesis (17, 18, 19). Polyamine metabolism and requirements are frequently dysregulated in cancer and other hyperproliferative diseases (18). For instance, high levels of ODC enzyme activity and polyamine levels were found in colon cancer (20) and breast cancer (21). In addition, the polyamine pathway is a downstream target for many oncogenes (18). This includes MYC, which regulates expression of ODC (22), and p53, which regulates expression of SAT1, a metaboliser of polyamines (23). Various therapeutic strategies have been developed to target polyamine metabolism, including direct inhibition of polyamine biosynthesis enzymes (e.g., DFMO) or by providing polyamine analogs (e.g., DENSpm), which can upregulate pathways that accelerate polyamine catabolism (23).
How are they regulated?
It may come as no surprise that polyamines, being largely translation-influencing molecules in nature, are regulated not at the transcriptional level, but at the level of translation. Outside of the two enzymes we know to be involved in the early stages of polyamine biosynthesis (ODC and AMD1), there are two others that are crucial due to the impact of their regulatory effects; ODC antizyme (OAZ) and antizyme inhibitor 1 (AZIN1).
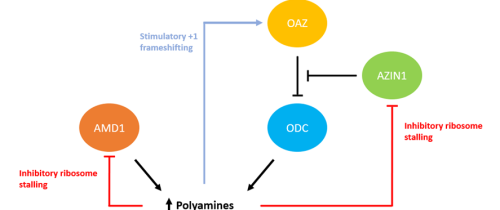
Figure 2: Polyamine regulatory network. Putrescine is produced by ODC, which itself is regulated by OAZ (antizyme) and AZIN1 (antizyme inhibitor). The expression of these genes are regulated at a level of translation. The production of AMD1 is also regulated at a translational level through a negative feedback mechanism.
OAZ
The main regulator of ODC is its antizyme, OAZ. It functions through its binding to ODC monomers, preventing their dimerization and subsequent enzymatic activity (24). OAZ expression is, however, intrinsically linked to the production and impact of polyamines. Full-length OAZ is coded for by two partially overlapping reading frames. At low polyamine concentrations, translation terminates at a stop codon at the end of the initial reading frame. However, at higher concentrations, a +1 frameshift occurs, resulting in a reading of the second frame, and production of the functional full-length protein (25). Through this process, a negative feedback loop is established, contributing to the regulation of polyamine biosynthesis.
AZIN1
However, the interactions between ODC and OAZ alone do not represent the full picture of polyamine regulation. AZIN1 is an antizyme inhibitor of OAZ, and is an early vertebrate homolog of ODC which has lost the ability to decarboxylase ornithine. Despite this, it still has a particularly strong binding affinity for OAZ, which drastically reduces the ability of OAZ to bind to ODC (26), thus degrading its inhibitory potential. This in turn encourages polyamine biosynthesis.
The regulation of AZIN1 also occurs at the translational level. Present in the 5’ leader region of this gene is an upstream conserved coding (uCC) region. This region is noted for the presence of a non-canonical initiation site, at which initiation is enhanced in the presence of high polyamine levels. This in turn reduces expression of the main ORF, thus lowering wider antizyme activity (27). It is hypothesised that ribosomes initiating at these uCC regions exhibiting stalling at a conserved PPW motif in the presence high polyamine levels, which leads to subsequent ribosomal queueing and reduced mORF translation (28).
AMD1
Adenosylmethionine decarboxylase, otherwise known as AdoMetDC or AMD1, catalyses the formation of the donor molecule required for the synthesis of spermine and spermidine. Unlike the ODC, OAZ and AZIN1, whose regulatory mechanisms are based off of their direct interactions with each other, AMD1 undergoes an autoregulatory process.
AMD1 mRNA contains a conserved uORF, located extremely proximal to the 5’ cap (~15 nucleotides downstream), encoding a peptide called MAGDIS. Similar to the uCC seen with AZIN1, increased polyamine expression increases the likelihood of stalling at this uORF, specifically at the termination codon. Due to its proximity to the 5’ cap, stalled ribosomes at this codon limit the ability of new pre-initiation complexes to bind, thus reducing ribosomal occupation and subsequent expression (29). Subsequently, polyamine synthesis is impaired.
Conclusions
Clearly, polyamines are key regulators of the process of translation. They are crucial molecules, essential for the key cellular functions of growth, embryonic development, differentiation, and proliferation. Accordingly, they have also been implicated in a number of translationally relevant diseases, such as cancer and ALS (30, 31). As such, investigation into polyamine regulation may yield developments in future treatments. Similarly, it is not inconceivable that knowledge of their mechanisms may be utilised in the development of mRNA therapeutics. While knowledge of their role in certain diseases is important, further research into their basic functionality will likely prove crucial for such treatments, and should be encouraged.
References
1. Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61(9):880-94.
2. Amarantos I, Xaplanteri MA, Choli-Papadopoulou T, Kalpaxis DL. Effects of two photoreactive spermine analogues on peptide bond formation and their application for labeling proteins in Escherichia coli functional ribosomal complexes. Biochemistry. 2001;40(25):7641-50.
3. Noeske J, Wasserman MR, Terry DS, Altman RB, Blanchard SC, Cate JH. High-resolution structure of the Escherichia coli ribosome. Nat Struct Mol Biol. 2015;22(4):336-41.
4. Konecki D, Kramer G, Pinphanichakarn P, Hardesty B. Polyamines are necessary for maximum in vitro synthesis of globin peptides and play a role in chain initiation. Arch Biochem Biophys. 1975;169(1):192-98.
5. Hershko A, Amoz S, Mager J. Effect of polyamines and divalent metals on in vitro incorporation of amino acids into ribonucleoprotein particles. Biochem Biophys Res Commun. 1961;5:46-51.
6. Atkins JF, Lewis JB, Anderson CW, Gesteland RF. Enhanced differential synthesis of proteins in a mammalian cell-free system by addition of polyamines. J Biol Chem. 1975;250(14):5688-95.
7. Gutierrez E, Shin BS, Woolstenhulme CJ, Kim JR, Saini P, Buskirk AR, et al. eIF5A promotes translation of polyproline motifs. Mol Cell. 2013;51(1):35-45.
8. Schuller AP, Wu CC, Dever TE, Buskirk AR, Green R. eIF5A Functions Globally in Translation Elongation and Termination. Mol Cell. 2017;66(2):194-205.e5.
9. Park MH, Wolff EC. Hypusine, a polyamine-derived amino acid critical for eukaryotic translation. J Biol Chem. 2018;293(48):18710-8.
10. Melnikov S, Mailliot J, Shin BS, Rigger L, Yusupova G, Micura R, et al. Crystal Structure of Hypusine-Containing Translation Factor eIF5A Bound to a Rotated Eukaryotic Ribosome. J Mol Biol. 2016;428(18):3570-6.
11. Ogasawara T, Ito K, Igarashi K. Effect of polyamines on globin synthesis in a rabbit reticulocyte polyamine-free protein synthetic system. J Biochem. 1989;105(2):164-7.
12. Hunter AR, Farrell PJ, Jackson RJ, Hunt T. The role of polyamines in cell-free protein synthesis in the wheat-germ system. Eur J Biochem. 1977;75(1):149-57.
13. Hetrick B, Khade PK, Lee K, Stephen J, Thomas A, Joseph S. Polyamines accelerate codon recognition by transfer RNAs on the ribosome. Biochemistry. 2010;49(33):7179-89.
14. Christian BE, Haque ME, Spremulli LL. The effect of spermine on the initiation of mitochondrial protein synthesis. Biochem Biophys Res Commun. 2010;391(1):942-6.
15. Landau G, Bercovich Z, Park MH, Kahana C. The role of polyamines in supporting growth of mammalian cells is mediated through their requirement for translation initiation and elongation. J Biol Chem. 2010;285(17):12474-81.
16. Landau G, Ran A, Bercovich Z, Feldmesser E, Horn-Saban S, Korkotian E, et al. Expression profiling and biochemical analysis suggest stress response as a potential mechanism inhibiting proliferation of polyamine-depleted cells. J Biol Chem. 2012;287(43):35825-37.
17. Elmets CA, Athar M. Targeting ornithine decarboxylase for the prevention of nonmelanoma skin cancer in humans. Cancer Prev Res (Phila). 2010;3(1):8-11.
18. Casero RA, Jr., Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6(5):373-90.
19. Murray-Stewart TR, Woster PM, Casero RA, Jr. Targeting polyamine metabolism for cancer therapy and prevention. Biochem J. 2016;473(19):2937-53.
20. Giardiello FM, Hamilton SR, Hylind LM, Yang VW, Tamez P, Casero RA, Jr. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 1997;57(2):199-201.
21. Manni A, Grove R, Kunselman S, Aldaz CM. Involvement of the polyamine pathway in breast cancer progression. Cancer Lett. 1995;92(1):49-57.
22. Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci U S A. 1993;90(16):7804-8.
23. Novita Sari I, Setiawan T, Seock Kim K, Toni Wijaya Y, Won Cho K, Young Kwon H. Metabolism and function of polyamines in cancer progression. Cancer Lett. 2021;519:91-104.
24. Kahana C. The antizyme family for regulating polyamines. J Biol Chem. 2018;293(48):18730-5.
25. Ivanov IP, Atkins JF. Ribosomal frameshifting in decoding antizyme mRNAs from yeast and protists to humans: close to 300 cases reveal remarkable diversity despite underlying conservation. Nucleic Acids Res. 2007;35(6):1842-58.
26. Ivanov IP, Firth AE, Atkins JF. Recurrent emergence of catalytically inactive ornithine decarboxylase homologous forms that likely have regulatory function. J Mol Evol. 2010;70(3):289-302.
27. Ivanov IP, Loughran G, Atkins JF. uORFs with unusual translational start codons autoregulate expression of eukaryotic ornithine decarboxylase homologs. Proc Natl Acad Sci U S A. 2008;105(29):10079-84.
28. Ivanov IP, Shin BS, Loughran G, Tzani I, Young-Baird SK, Cao C, et al. Polyamine Control of Translation Elongation Regulates Start Site Selection on Antizyme Inhibitor mRNA via Ribosome Queuing. Mol Cell. 2018;70(2):254-64.e6.
29. Raney A, Law GL, Mize GJ, Morris DR. Regulated translation termination at the upstream open reading frame in s-adenosylmethionine decarboxylase mRNA. J Biol Chem. 2002;277(8):5988-94